The RMU medical device – Defibtech’s RMU Recall
KBD Attorneys are litigators and trial lawyers. We are leaders in medical device litigation. KBD investigates many medical devices, including heart related medical devices. Impella is a recent one, as is the Defibtech discussed below. Defibtech removed the RMU medical device from the market.
Defibtech, LLC, Removes the RMU Medical Device – the RMU-2000 ARM XR Chest Compression Device – due to Risk of Device Stopping Compressions
To live, the heart needs to beat at a constant rhythm. When patients’ hearts stop, hopefully someone is there to perform CPR immediately. The body needs the oxygen rich blood from the heart. A person will die without these live saving comprehensions to the heart. That’s what makes the RMU medical device so important to us.
A device that constantly pumps the heart can be crucial for some patients whose heart can not beat on their own. Manual chest compressions can vary in quality depending on the strength, endurance and technique of the person performing them. Automatic devices can provide consistent, high-quality compressions. This ensures the best possible chance of survival. Debitech hoped the RMU medical device was one such product.
Debitech’s RMU medical device, when it works properly, can increase the survival rate of patients. Defibtech’s RMU-2000 ARM XR chest compression device is under scrutiny. The US Food and Drug Administration (FDA) issued a Class I recall (the most serious type) for the rmu medical device. The affected devices are being removed from the market after several reported injuries and a death of a patient.
What is the Problem with the RMU medical device?
The FDA announced that the RMU medical device, a chest comprehension device, has had problems with the motor. These problems with the RMU medical device may cause it to stop its automatic compressions. When the RMU medical device stops compressions, it also stops the oxygen from circulating in the body. This can lead to a delay in therapy, injuries, and death. Defibtech reported one injury and one death by the July RMU recall.
Imagine someone goes into cardiac arrest, a child at a sports event, a loved one at home, a patient in a pre-hospital setting. A family, organization, or facility took precautions and purchased a Defibtech RMU-2000 ARM XR Chest Compression Device. A good Samaritan springs into action to perform CPR. They tire waiting for emergency services. They use the RMU medical device. Onlookers hope and pray the near lifeless body to resuscitate. Then suddenly, the RMU medical device stops working. You can almost hear the “flat-line” sound in your head. After one injury and a death, this fear gave rise to the RMU recall.
The Debitech RMU Recall Affects the Following RMU Medical Devices:
- RMU-2000 Chest Compression Module
- Product Name: RMU-2000 ARM XR Chest Compression Device
- Unique Device Identifier (UDI)/: UDI-DI: 00815098020812, 10815098020819
- Serial Numbers: See full list at the FDA RMU recall site.
Defibtech distributed these RMU recall devices in Connecticut, Florida, New Jersey, South Carolina and Tennessee. This is what the RMU medical device looks like:
How Does This RMU Medical Device Withdrawal Affect Patients and Their Families?
The RMU recall impacts people across the U.S. Thousands of families have had loved ones die of heart attacks, cardiac arrest, and other heart issues. Unfortunately, many do not have defibrillators available to keep their hearts beating. In many situations, these failed hearts depend on the people around them and their ability to perform manual compressions correctly and consistently. 70% of heart attack victims die without AED machines. This may be in part because manual compressions can be unreliable.
Patients and their families rely on these automatic devices to live, because they are reliable. Knowing that their loved one is receiving the best possible care, with consistent and accurate compression, can offer reassurance during a highly stressful and emotional time. Families should be able to trust that their loved one is receiving the best treatment.
The RMU recall suggests Defibtech failed to ensure that patients were safe and that their families were at ease. The motor problems with the RMU medical device provide a lack of trust for both the patients and their families. The RMU recall of the RMU medical device was a good reaction, but now we wonder about missed proactive steps that could have prevented injury or death.
Defibtech’s Response
Defibtech issued an Urgent Medical Device Safety Removal letter in July after reports of the defect. There was one injury and one death preceding the RMU recall. In the RMU recall letter, the company identified 211 RMU medical devices that people should stop using and return to Defibtech immediately. The RMU recall remains in place and is a true market withdrawal or the RMU medical device.
RMU Medical Device Lawsuits
If someone died because the RMU medical device stopped working, they may have a legal claim. Many heart-related medical devices are PMA devices. That means they got to the market through FDA’s pre market approval process. Accordingly, there are complex legal doctrines at issue, including one called preemption.
By comparison, the RMU device subject to the RMU recall got to market via the FDA clearance process. Specifically, FDA cleared the device via the 510(k) process in 2021. This chest compression device did not face the same scrutiny by FDA. Preemption likely does not apply. That makes product liability claims for a product liability lawyer much easier.
The RMU Still for Sale?
We appreciate that not everyone hears about product recalls when they happen. We represent people who never heard a product they had was the subject of a recall. Consequently, we try to maintain our pages and post about important recalls like the RMU medical device.
Despite this recall, it seems the RMU is still for sale. Here are some results we found with a simple research.
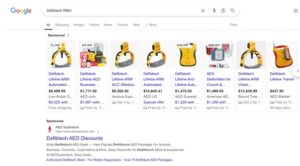
Search results on Google for RMU (Jan. 2025.)
Perhaps it’s a different model or otherwise not subject to the RMU recall. We hope folks heed the recall.
How KBA Can Help Families Impacted by the RMU Medical Device?
At our law firm, we litigate failed medical device cases. We investigate product failures like the RMU recall.
If a RMU medical device injured you or a loved one, contact our product liability lawyers. You may not know if an RMU medical device was used, or even if, it was part of the RMU recall. If a loved one died after a device was used to help with CPR, call us (855-KBA-LAWS (522-5297)). We can provide an initial consultation, free of charge, to evaluate the details of your case and help you seek compensation for physical and emotional injuries from the RMU medical device if applicable.


