BioZorb Implant Complications BioZorb Lawsuit attorneys,” or “BioZorb Marker Recall Information
The BioZorb Recall
After recalls in March 2024 and warnings due to safety concerns and injuries, FDA told doctors and patients – do not use BioZorb in late 2024. Since coming to market in 2015, doctors and patients trusted BioZorb to help. Now we know BioZorb likely injured many women. We provide information and details here.
Patients experiencing BioZorb complications should speak with their healthcare providers. Anyone who had the BioZorb implant should contact KBA. Our product liability attorneys can bring a BioZorb lawsuit or otherwise advise you concerning the BioZorb recall. Call 855-KBA-LAWS (522-5297) to see if we can help you.
Now, here’s the BioZorb story from getting to market to a true recall, a market withdrawal in 2024.
BioZorb Background – How Did BioZorb Get on the Market?
The BioZorb story goes back to at least 2015. In June that year, Focal Therapeutics, submitted a 510(k) premarket notification of intent to market the device, which FDA cleared. The BioZorb Marker, its official name, is indicated for radiographic marking of sites in soft tissue.
Put simply, that means placing a marker – a material or object – within the body to identify a specific location for medical imaging purposes. This marker is typically made of a material that is visible on X-rays or other imaging technologies, such as titanium or other radiopaque substances. A marker is usually used to:
- Guide Medical Procedures: For example, during radiation therapy, the marker helps ensure that treatment targets the precise area where surgery or a tumor was located.
- Monitor Healing or Recurrence: Markers allow radiologists to assess the surgical site over time, checking for complications, recurrence of disease, or changes in the tissue.
- Provide Reference Points: In cases where multiple imaging studies are needed, markers serve as consistent reference points, reducing uncertainty and improving diagnostic accuracy.
The company got the BioZorb implant on the market via the 510(k) process. Learn more about that on our medical device page.
What is the BioZorb Marker?
The BioZorb Marker is a surgical marker designed for breast-conserving surgeries like lumpectomies. It was an implantable radiopaque marker comprised of: 1) a bioabsorbable PLA (polylactic acid) component that should resorb completely in one or more years; and 2) a permanent component (titanium). It was “made of six clips to mark the surgical site of tissue removal in three dimensions.” The BioZorb Marker was for single use.
Recent reports of the titanium marker highlight serious complications, including infections, pain, seromas, and even device migration, leading to a Class I recall—the most critical type issued for medical devices.
The Dangers of BioZorb – Dealing with BioZorb Device Complications and Removal – The BioZorb Recall
The First BioZorb Recalls – Spring of 2024
The U.S. Food and Drug Administration (FDA) classified the initial recalls of Hologic’s BioZorb Marker as a Class I recalls. Class I is the most serious type. It indicates a high risk of serious injury or death.
Hologic’s recall on March 13, 2024, affected 53,492 devices distributed between April 29, 2019, and April 1, 2024. The BioZorb titanium marker recall arose because of complications and reported adverse events. Complaints and BioZorb marker complications include reports of pain, infection, rash, device migration, device erosion, seroma, discomfort, or other complications from feeling the device in the breast, and the need for additional medical treatment to remove the device. By then, there were 71 reported injuries and no reports of death.
Many people think that means they took the product back, removed it from the shelves. But that’s not what happened, at first. Rather, the Biozorb recall instructed patients to speak with healthcare providers if they had averse effects. It was more a notice of the possibility and a call for reports.
BioZorb Withdrawal October 2024
On October 10, 2024, the company, Hologic, recalled BioZorb .In October though, FDA warned the community – do not use BioZorb. The case against this product couldn’t be any more clear. Hence, our focus on the BioZorb lawsuit.
What BioZorb Implants Are Included?
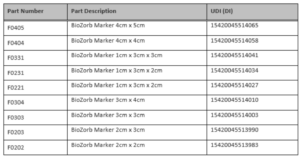
List of BioZorb products from Hologic.
BioZorb Complications: Breast Biopsy Marker Side Effects
The BioZorb recall arose from numerous reports of complications and adverse events associated with the BioZorb Marker, including:
- Pain
- Infection
- Rash
- Device migration and erosion
- Seroma (fluid buildup)
- Discomfort
- Necessity for additional medical treatment to remove the device
Some reports found eroding through the nipple. As of now, 71 BioZorb titanium marker injuries have been reported, although no deaths have been associated with the device (FDA) (FDA) (MPO Magazine).
Hologic identified the following patient complaints describing BioZorb implant complications/adverse events, BioZorb symptoms that “include serious injuries such as pain, infection, rash, device migration, device erosion, seroma, discomfort, and/or other complications from feeling the device in the breast, and in some limited instances, additional medical treatment.”
BioZorb Usage and Distribution
The BioZorb Marker is an implantable radiographic marker used to mark soft tissue, such as breast tissue, for future medical procedures, including radiation therapy. The device has a permanent titanium component and a resorbable plastic component designed to dissolve over time (Medical Device Developments) (Health Imaging).
BioZorb Complications: Potential Harm from BioZorb and People Affected
Given the number of BioZorb units sold, the potential impact is significant. The BioZorb recall affects a wide range of healthcare professionals, including radiologists, surgeons, and oncologists, who use these markers in breast cancer procedures. Patients with implanted BioZorb markers are at risk, and those undergoing systemic cancer treatments may experience delays due to complications from the marker. For more on this topic, read here:
Understanding BioZORB: Benefits, Complications, and Concerns
Lack of Warnings Concerning BioZorb
Many patients with the implanted BioZorb titanium markers are not aware that they are at risk or know the possible side effects to expect. The device lacks important warnings that consumers and their healthcare professionals should be aware of before the implanting the device. Here is some additional information:
Biozorb Risk Mitigation and Recommendations
Hologic has issued safety notifications to all affected BioZorb customers, urging patients to contact their healthcare providers if they experience any adverse events. Healthcare providers are advised to continue monitoring patients with the implanted device and report any complications to Hologic and the FDA’s MedWatch Adverse Event Reporting program (FDA Access Data) (Medical Device Network).
Now, as of October 2024, FDA says not to use BioZorb.
In summary, the recall of the BioZorb Marker highlights the need for vigilant monitoring and reporting of medical device complications to ensure patient safety and mitigate potential harm.
KBA is Litigating the BioZorb Lawsuit
The BioZorb lawsuit is moving ahead quickly. The BioZorb lawsuit is gaining more traction since the BioZorb recall. The primary situs for the BioZorb marker cases is in federal court in Massachusetts.
Sometimes referred to as the titanium breast marker lawsuit, these cases are before a court in Massachusetts (that may not be your only option, speak to our attorneys to learn more about your specific case.)
KBA has an attorney licensed in Massachusetts where this litigation resides. Our partner heading the litigation has vast medical device experience including trying medical device cases. The legal team working on Biozorb is skilled at caring for clients and getting cases worked-up quickly. Thus, KBA is well-positioned to help people BioZORB injured.
Michael Appel is an attorney in the state where the company is located, Massachusetts. He has litigated medical device cases, complex medical malpractice cases, and more for decades. A true trial lawyer, Michael has led complex litigations and mentors KBA’s attorneys. Michael tried device cases and will be with us if and when we have a Biozorb trial.
Partner Robert Price has litigated many medical device cases. He recently tried one in NJ. He hails from a preeminent firm and has taken the reigns of the medical device team here at KBA. Our firm is passionate about fighting this matter. Robert investigates each Biozorb case and is helping lead the BioZorb lawsuit in MA.
Compassionate Counselors at Law When BioZorb complications Arise
Having great lawyers is only one part of the equation. Many of our clients never needed a lawyer before, or even if, not a product liability attorney. They are uncertain about what to do.
The men and women working on the BioZorb implant team understand the BioZorb titanium marker complications, the BioZorb recall, and the strategy behind BioZorb lawsuits and other strategies. They are compassionate, caring people who want to help. Expertise, experience, and empathy are what set our BioZorb implant team apart.
The FDA BioZorb Warning Letter
After the BioZorb recall and the BioZorb withdrawal, FDA sent a warning letter about the Breast Biopsy Marker BioZorb. On December 19, 2024, FDA contacted Hologic, Inc.
The FDA cited serious concerns about their management of the BioZorb marker, including: 1) failure to report adverse events; 2) inadequate post-market surveillance; and 3) manufacturing process deficiencies. This is significant.
Hologic’s failure to comply with Medical Device Reporting (MDR) regulations left patients and healthcare providers uninformed about potential risks, bolstering failure-to-warn product liability claims and potentially bypassing. Learn the full details here:
Latest Developments in the BioZorb Lawsuits
We continue filing lawsuits on behalf of our clients. Our BioZorb lawyers are pleased with how the litigation is unfolding. We reported previously about upcoming trials.
As we learn more, we become more confident in our pursuit of justice for women who had a BioZorb implant. Here’s a recent update:
Important Victory for Patients in BioZorb Medical Device Litigation
Stay Informed about the BioZorb Implant and Hologic BioZorb Lawsuits
KBA continues informing BioZorb patients about the latest developments about Hologic BioZorb.
A woman who had a BioZorb titanium marker can file a BioZorb implant lawsuit. Many plaintiffs filed BioZorb lawsuits in the MDL in Massachusetts. In February 2025, the judge ruled against one of the plaintiffs.
In a product liability lawsuit like the cases concerning the BioZorb recall, plaintiffs often assert a failure to warn claim. They allege the company knew or should have known about risks concerning BioZorb implant complications that were not in the product’s label or labeling. Put simply, they did not warn the woman or her doctor about specific risks.
To succeed on BioZorb marker failure to warn claims, the plaintiff must provide admissible evidence that if the company warned, disclosed the risks, it would have made a difference. If the doctor would have used the device regardless of the warning, there is likely no failure to warn claim. That’s what happened in this particular case. So we have a defense win in this case where the Court dismissed the failure to warn claim.
This decision underscores how hard these cases are. Plaintiff treater depositions are truly a science and an art with serious consequences.
Recent KBA BioZorb Implant Complication and Holigic BioZorb Lawsuit Information Updates
Here are some recent blog posts:
The Dangers of BioZorb – Dealing with BioZorb Device Complications and Removal – The BioZorb Recall
BioZORB Litigation Ruling: A Turning Point in Medical Device Safety
Understanding BioZORB: Benefits, Complications, and Concerns
BioZORB Litigation Ruling: A Turning Point in Medical Device Safety
The Dangers of BioZorb – Dealing with BioZorb Device Complications and Removal – The BioZorb Recall
CONTACT KBA TODAY – What Can KBA Do For You Concerning the BioZorb Implant?
We litigate medical device cases at our law firm. Our approach to working with women and their families suffering BioZorb implant complications, or just concerned about the device at this stage, is reflected in our E3 approach – expertise, experience, and empathy. Our product liability attorney takes the role of counselor at lawyer to heard and brings decades of experience and in-depth knowledge to each case. We support people who are nervous about how they may be affected in the future, and individuals who have suffered injuries, throughout the entire process.
Patients with cancer are near-and-dear our hearts. Cancer impacts all of our families. As lawyers, we have worked on other kinds of breast cancer lawsuits including medical malpractice, pharmaceutical, and medical device cases. The Biozorb markers case fits perfectly well with our experience.
Our skilled attorneys are committed to helping you navigate the complexities of personal injury law and claims related to BioZorb. If you or a loved one has suffered harm from the BioZorb implant at issue in the BioZorb recall – contact us immediately, strict deadlines apply.
855-KBA-LAWS (522-5297)

