Why Do We Sue Medical Device Companies?
I served the medical device industry for many years before attending law school. Me and my family have benefited from medical devices, and as a business owner and as a technophile (a technology enthusiast) I want nothing more than continued progress in the medical technology marketplace.
When I began practicing law, I was a defense lawyer. It took a long time to come around to the plaintiffs’ side because of my good experiences working with various industries and because I had heard (what turned out) to be overblow hype and propaganda about plaintiffs’ lawyers and “runaway” juries. I only wanted to get involved if there was truly bad conduct and I was surprised to learn there was.
Time and time again, it is the same story – medical device companies either bury their heads in the sand by failing to look for risks associated with the design of their product, or they learn of risks associated with their devices, but bury them rather than warn the public about them. Most of the time, the companies do the right thing.
However, some companies let fear and greed get the better of people and rather than go down the right path, they diverge and make bad choices. We’ve all been there. The key is to face it head on and try to make it right, and that’s at its core why we sue medical devices companies; we bring medical device lawsuits when companies fail to follow the rules and people are injured as a result.
Medical devices are dangerous when companies fail to follow the rules regarding risk management.
“Safety and quality are non-negotiables in the medical devices industry.” States the International Organization for Standardization (“ISO”). To try to ensure that patients receive only safe and good quality devices, the medical device industry is regulated by the FDA. Specifically, the Center for Device and Radiological Health (CDRH) at the FDA oversees medical device companies.
One of the things the FDA does is write regulations for medical devices. See, e.g.,
which discuss regulations for medical devices. There are several other industry standards that apply to medical device companies as well.
Many of the industry standards that come into play in a lawsuit concern identifying and resolving risks to the patients. The FDA set forth regulations that medical device companies must follow to prevent injuries. These regulations are designed to help keep patients safe. One such set of FDA regulations that the attorneys at KBD often focus on in medical device lawsuits is the Quality Systems Regulation (“QSR”). (This is actually a favorite topic for Attorney Browne who publishes and speaks on this topic, and often works with leading experts to address in litigation.)
The QSR concerns quality systems, which medical device companies use to help “ensure that their products consistently meet applicable requirements and specifications.” From the FDA, Medical Devices. These regulations arose based in part because of “findings that a significant proportion of device recalls were attributed to faulty design of product.” Id. The FDA developed them by looking around the world at various standards for making and managing medical devices throughout their entire product lifecycle.
In large part, the important part this system focuses on how companies should identify and control risks or hazards associated with their medical devices. That is our major focus – how can this device hurt people and what did the company do to prevent that from happening?
Attorneys bring medical device lawsuits when companies fail to address risks to patients.
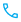
Risk is something we manage every day in our lives, but perhaps don’t think about much. Within medical device companies, managing risks is critical to patient safety. I like to think about risk as an iterative process – a continuous system that involves looking for risks, studying them, putting things in place to prevent the risks, and continuing to watch to make sure the fixes work and to find new risks that need to be eliminated. Here’s a good visual from Google Images:
All industries have some system like this for identifying and controlling risks, from the auto to the service industry. There are general principles that apply to all industries and then within each there are industry specific standards. These standards, or rules if you will, guide how people within the company should handle problems that arise with their products. Companies use them to develop internal procedures and processes.
When medical device companies follow the rules, they learn of risks to patients by studying their product – who uses it, how, why, and what happens when they do? They also learn of risks through complaints about the product and complaints that involve injuries caused by the product, or adverse events.
When medical device companies receive information that their products may injury people or malfunction in a way that presents risk, they must act. They must figure out why someone got hurt and do something to prevent it from happening again. They must take corrective and preventive actions. They do so by implementing risk controls – things designed to eliminate or lessen the occurrence of a risk.
There are many ways to control risks, including redesigning a product and providing instructions and warnings to make sure the product is used properly, or not at all under certain circumstances. It is critically important that medical device companies not only identify risks, but also implement controls, and that they monitor those risk controls to be sure they eliminate or at least reduce, the risk, especially over time.
When medical device companies do not follow the process and people get hurt, KBD is there to help.
Justin Browne, Esq